Heat Capacity:
It is the amount of heat required to raise the temperature of a body by 1 K (or 1 degree c)
Symbol for heat capacity – C
Unit – Jk-1 or J°C-1
Where is the heat energy absorbed and is the change In temperature.
Specific Heat Capicity:
It is the ammount of heat required to raise the temperature of 1 kg of the substance by 1K or Jkg-1 °c
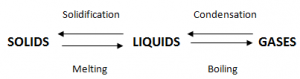
Melting:
Melting is the change of state of an object from solid to liquid without a change in temperature.
During melting the temperature remains constant at the melting point. Heat is absorbed by the substance.
Solidification:
Solidification is the change of state an object from liquid to solid without change in temperature.
In this process heat is released from the object.
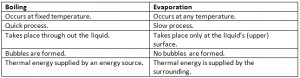
Differnce Between Boiling And Evaporation:
Why Evaporaton Causes Cooling:
It requires thermal eneergy from the surroundings. Thermal energy from your body helps the water on the skin to evaporate. When water evaporates it takes away the thermal energy from the surface of your skin.
Latent Heat:
Latent heat is the energy absorbed or released during a change of state of an object having mass of 1 kg.
Latent Heat Of Fusion:
Latent heat of fusion (lf) of a solid, is the heat required to change it from solid to liquid state or vice versa. Without any change in the temperatue.
lf = Joule
As mass increases, the latent heat increases.
Specific Latent Heat Of Fusion:
The specific latent heat of fusion (Lf) of a solid substance is the heat required to change 1kg of it from solid to liquid or vice verca without any change in the temperature.
Lf = m (kg) × lf (Jkg-1)
Latent Heat Of Vapourization:
Lv of a substance is the heat required to change it from liquid to vapour state or vice verca from water vapours to liquid , without any change in temperature.
Lv = Joules
Specific Latent Heat Of Vapourization:
Lv of a substance is the heat required to change 1 kg of it from liquid to vapour or viceversa without any change in temperature.
Lv = m × lv
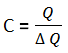
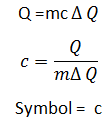
No comments:
Post a Comment