Pressure is defined as the force acting per unit area.
SI unit: Pascal (P) or Nm-2
Pressure In Liquids:
Pressure in liquids is given by the formula:
Pressure = density × gravity × height
Pressure increases with the depth and density of the liquid.
While calculating pressure in liquids height should be always measured from the bottom.
Pressure In Gases:
Pressure in gases is due to the bombardment of molecules on walls of container and it can be measured by manometers.
Barometer:
It is an instrument for measuring atmospheric pressure. There are two types of barometers mercury barometer and water barometer
Boyle’s Law:
Boyle’s law states that the pressure exerted by gas is inversely proportional to the volume provided the temperature remains constant.
Therefore:
P1v1 = p2v2
Whenever temperature increases the volume increases
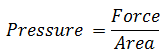
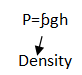
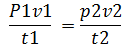
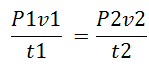
No comments:
Post a Comment