The kinetic model of matter is made up of tiny particles which exist as atoms, molecules and ions.
The particles are always in continuous, random motion and hence posses kinetic energy. The kinetic energy of a particle increases with temperature and pressure. At fixed temperature lighter particles moves faster.
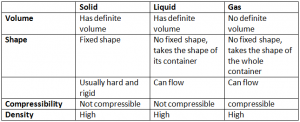
Properties Of Solid Liquid And Gases:
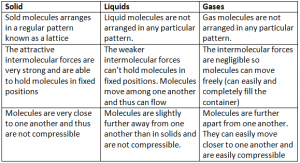
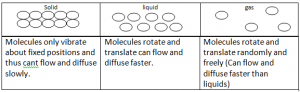
Molecular Model Of The States Of Matter:
Brownian Motion:
Browninan motion is the random or irregular motion of smoke particles in air or pollen grains in water.
Brownian motion is caused by the bombardment of air molecules on smoke particles.
Brownian motion of tiny particles is caused by the continual bombardment of fast moving gas or liquid molecules on the tiny particles.
The smoke particles will be seen as moving in random direction rather than in fixed direction.
Relationship of Temperature and Molecules:
At higher temperature molecules have greater speed
Therefore
As temperature increases the motion of molecules increases
Pressure In Gases:
Pressure of a gas is due to the collision of gas molecules with the walls of the container..
Air inside the container exerts an outward pressure equal to the atmospheric pressure outside.
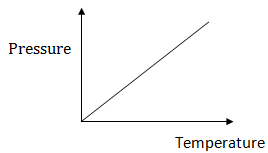
Relationship Between The Temperature And Pressure Of A Gas:
Temperature is directly proportional to Pressure
Therefore as temperature increases the Pressure increases and vice versa if temperature decrease the pressure decreases.
As the container containing gas is heated, the gas particles will gain kinetic energy and move faster. When they will move faster they will hit the walls more often and more force will be exerted on the walls per unit area.
No comments:
Post a Comment