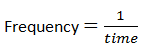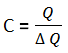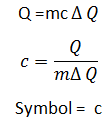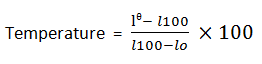For the past papers click here:
http://papers.xtremepapers.com/index.php?dir=CIE/Cambridge%20International%20O%20Level/Physics%20%285054%29/
Friday, 19 May 2017
Light
Light is a form of energy. The speed of light is 3 × 108 ms-1 and it travels at a constant speed. It can reach the earth from the sun in 8 minutes.
Human eye can detect it in a range of 7 colours from red to violet which forms a spectrum.
Rectilinear Properties Of Light:
- It states that light travels in straight line.
- It can’t bend around corners and can only travel straight
- The path along which light travels is known as light ray. Arrows are added to indicate the direction.
Combination of rays forms a beam. There are three types of beams:
Parallel:
Converging:
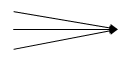
Diverging:
Luminous Objects: These are those objects that give out light. E.g.TV, Sun, Light bulbs
Non-Luminous Objects: These are those objects that do not give out light.
We can see them as the object reflect light from a luminous object nearby into our eyes.
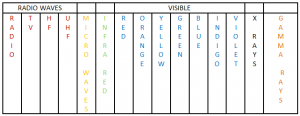
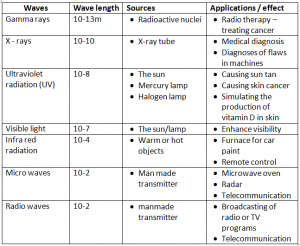
Electro Magnetic Spectrum
The Electro Magnetic Spectrum contain different types of waves, some of which are visible to human eye.
Properties Of The Electro Magnetic Waves:
- They all are transverse waves.
- They transfer energy from one place to another.
- They can travel through vacuum, no need of medium.
- They all travel at 3 × 108 ms-1 in a vacuum.
- They all travel with the same speed in air.
- They all obey the laws of reflection and refraction.
Long wavelength Short wavelength
Low Frequency High Frequency
Sources And Applications Of Electro Magnetic Waves:
Remember this : Ronald Mcdonald Is Very Ugly eXcept Gary
General waves properties
Wave:
A wave is a phenomenon, in which energy is transferred through vibrations.
Properties:
- The source of any wave is vibration
- Waves transfer energy from one point to another.
- In waves, energy is transferred without the medium being transferred.
How Waves Are Formed:
There are two ways to form a wave.
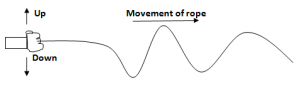
Waves Motion On A Rope:
- Fixing one end of the rope to a wall and moving the other end up and down
- These up and down movements produce vibrations.
- The rope waves travel towards the wall while the rope itself moves up and down.
- The kinetic energy from the up and down movement is transferred from one point to another.
- The rope itself however doesn’t move from one end to another.
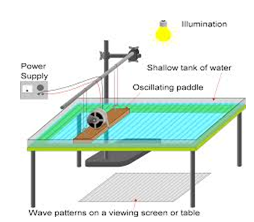
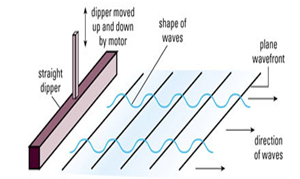
Water Waves In A Ripple Tank:
- Water waves can be generated using a ripple tank
- A small dipper moves up and down
- The water particles at the surface are made to move up and down spreads to other parts of the water surface.
- The kinetic energy is thus transferred to the water molecules at the surface.
- These water molecules in turn transfer to the neighboring water molecules.
- Though energy is passed but the water itself doesn’t move the dripler to the edges.
Types Of Waves:
Waves are of two types Transverse and Longitudinal.
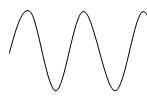
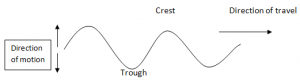
Transverse Waves:
- Transverse waves are waves that travel in a direction perpendicular to the direction of vibration.
- Displacement of particles in transverse waves is perpendicular to the direction of travel of wave motion.
- The highest point reached by a vibrating particle in a transverse wave is called crest or peak while the lowest peak is trough.
- e.g. water, rope, electromagnetic waves
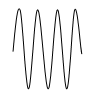
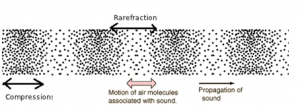
Longitudinal Waves:
- Longitudinal waves are waves that travel in a direction parallel to the direction of vibration.
- Displacement of the particles in longitudinal waves is in line with, or parallel to the direction of wave motion.
- e.g. sound waves
Compression: Section where the vibrating particles in a longitudinal wave are closest together.
Reflection: Section where the vibrating particles are furthest apart.
Describing Waves:
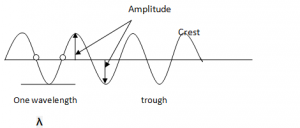
Crests and Troughs: These are the highest and the lowest points of a transverse wave, respectively. For longitudinal waves, the terms compression and rarefactions are used.
Phase: Any two points are said to be in phase when they move in the same direction and have the same speed and in the same displacement from the rest position (e.g. These two points)
Wavelength( λ): This is the shortest distance between any two points in a wave that are in phase such as two successive crests or troughs. The SI unit is metre (m).
Amplitude: This is the maximum displacement from the rest or centre position. It is the height of crest or trough measured from the rest position.
Period (t): Time taken for one point on the wave to complete one oscillation. This can also be written as the time taken, to complete one wave.
SI Unit is seconds(s)
On a displacement time graph one period = 1 sec
Frequency(f): This is the number of complete waves produced per second.
The SI unit is Hertz.
Wave Speed (V): The distance of the wave moved in one second in the medium.
V = f × λ
SI unit: metre per second (ms-1)
Wavespeed = frequency × wavelength
Wave Front:
It is the imaginary line on a wave that joins all points that are in same phase. It is usually drawn by joining down all the crests.
Plane dipper can produce plane waves in a ripple tank. Thus plane Wave fronts are seen.
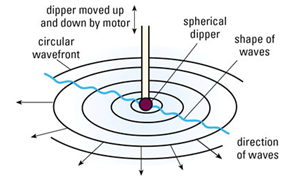
Spherical dipper on the other hand can provide circular waves in a ripple tank. These types of waves have circular Wave fronts.
Thermal properties of matter
Heat Capacity:
It is the amount of heat required to raise the temperature of a body by 1 K (or 1 degree c)
Symbol for heat capacity – C
Unit – Jk-1 or J°C-1
Where is the heat energy absorbed and is the change In temperature.
Specific Heat Capicity:
It is the ammount of heat required to raise the temperature of 1 kg of the substance by 1K or Jkg-1 °c
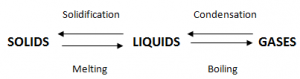
Melting:
Melting is the change of state of an object from solid to liquid without a change in temperature.
During melting the temperature remains constant at the melting point. Heat is absorbed by the substance.
Solidification:
Solidification is the change of state an object from liquid to solid without change in temperature.
In this process heat is released from the object.
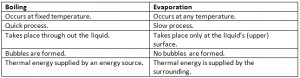
Differnce Between Boiling And Evaporation:
Why Evaporaton Causes Cooling:
It requires thermal eneergy from the surroundings. Thermal energy from your body helps the water on the skin to evaporate. When water evaporates it takes away the thermal energy from the surface of your skin.
Latent Heat:
Latent heat is the energy absorbed or released during a change of state of an object having mass of 1 kg.
Latent Heat Of Fusion:
Latent heat of fusion (lf) of a solid, is the heat required to change it from solid to liquid state or vice versa. Without any change in the temperatue.
lf = Joule
As mass increases, the latent heat increases.
Specific Latent Heat Of Fusion:
The specific latent heat of fusion (Lf) of a solid substance is the heat required to change 1kg of it from solid to liquid or vice verca without any change in the temperature.
Lf = m (kg) × lf (Jkg-1)
Latent Heat Of Vapourization:
Lv of a substance is the heat required to change it from liquid to vapour state or vice verca from water vapours to liquid , without any change in temperature.
Lv = Joules
Specific Latent Heat Of Vapourization:
Lv of a substance is the heat required to change 1 kg of it from liquid to vapour or viceversa without any change in temperature.
Lv = m × lv
Temperature
Teperature:
It is the degree of hotness or coldness. As it refers to how hot or cold the object is .
Heat:
Heat refers to the amount of thermal energy that is being transferred from a hotter to a cooler region.
Constructing A Thermometric Scale:
- Choose a thermometric substance.
- Mercury or alcohol
- Select two fixed points.
- Ice point – as the upper fixed point.
- Steam point – as the lower fixed point.
- Divide the temperature range between the two fixed points into equal divisions.
Ice point (lower fixed point):
This is the temperature of pure melting ice at a pressure of one standard atmosphere. It is assigned a value of 0°C
Steam point (upper fixed point)
This is the temperature of steam from water boiling at a pressure of one standard atmosphere. It is assighned a value of 100 °C
Centigrade Scale:
A Temperature scale that defines the freezing points of water as 0 °Cand the boiling points of water as 100 °C
Determination Of Ice Point :
- Immerse the bulb of the thermometer into a funnel containing pure melting ice.
- The mercury level in the stem should be just above the ice.
Determination Of Steam Point:
Insert the thermometer into the apparatus. The bulb of the thermometer should be above the boiling water.
Manometer is used to ensure pressure inside the apparatus is same as the atmospheric pressure outside.
Measuring tempereature using centigrade scale:
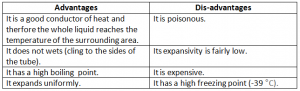
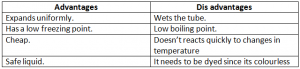
Advantages And Disadavantages Of Using Mercury As A Thermometric Liquid:
Advantages And Disadvantages Of Using Alcohol As A Thermometric Liquid:
Responsiveness:
This measures how quickly the thermometer can register changes in temperature.
Sensitivity:
This measure the amount of changes in the thermometric property (e.g. length of mercury column) per unit change in temperature.
Range:
This denotes the minimum and maximum temperature that the thermometer can measure.
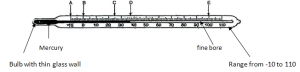
Laboratory Thermometer:
Mercury is contained in a thin walled glass bulb:
Thin wall of the glass bulballows conduction of heat quickly through the glass(poor conductor) to the liquid.
The bulb is made small to contain a small amount of liquid:
A smal amount of liquid responds more quickly to changes in temperature.
The bore of the capillary tube is fine and uniform:
The fine tube allows a noticeable movement of the liquid column for a small change in temperature. (good sensitivity).
Uniform tube ensures even expansion of liquid.
The walls of the long tube above the bulb are made thick:
This acts as a magnifying glass for easy reading of the mercury thread in the stem.
The size of the thermometer is relatively small:
The small size allows it to be portable and cheap to produce.
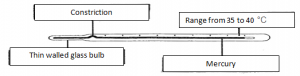
Clinical Thermometer:
The scale is limited to a small range of between 34 to 42:
Since the normal body temperature is 36.9 the short range allows for greater accuracy and the stem can be made responsibally short
Narrow constriction:
It prevents mercury from flowing back into the bulb by breaking the mercury thread at the constriction.
Thermocouple Thermometer:
It consists of two wires made of different metals such as iron and copper. The ends of wires are joined together to form two junctions.
When two junctions are at different temperature e.g. one is hot and the other is cold. An emf is produced.
The greater the change in temperature the greater the emf is produced across the two junctions.
Advantages:
- Large temperature range (-200 to 1500 ).
- Very responsive to rapidly changingn temperature due to its low thermal capacity, due to its small mass and metals are good conductors of heat.
- Output is elctrical signal therefore can be connected to a suitable electrical equipment for checking rapid or sudden temperaturechanges.
Subscribe to:
Comments (Atom)